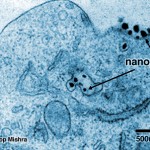
Those tiny black dots are nanobots delivering a lethal blow to a cancerous cell, effectively killing it.
Look close. You may be staring at the end of cancer.
Those tiny black dots are nanobots delivering a lethal blow to a cancerous cell, effectively killing it. The first trial on humans has been a success, with no side-effects:
It sneaks in, evades the immune system, delivers the siRNA, and the disassembled components exit out.
Those are the words of Mark Davis, head of the research team that created the nanobot anti-cancer army at the California Institute of Technology. According to a study to be published in Nature, Davis’ team has discovered a clean, safe way to deliver RNAi sequences to cancerous cells. RNAi (Ribonucleic acid interference) is a technique that attacks specific genes in malign cells, disabling functions inside and killing them.
The 70-nanometer attack bots—made with two polymers and a protein that attaches to the cancerous cell’s surface—carry a piece of RNA called small-interfering RNA (siRNA), which deactivates the production of a protein, starving the malign cell to death. Once it has delivered its lethal blow, the nanoparticle breaks down into tiny pieces that get eliminated by the body in the urine.
The most amazing thing is that you can send as many of these soldiers as you want, and they will keep attaching to the bad guys, killing them left, right, and center, and stopping tumors. According to Davis, “the more [they] put in, the more ends up where they are supposed to be, in tumour cells.” While they will have to finish the trials to make sure that there are no side-effects whatsoever, the team is very happy with the successful results and it’s excited about what’s coming:
What’s so exciting is that virtually any gene can be targeted now. Every protein now is druggable. My hope is to make tumours melt away while maintaining a high quality of life for the patients. We’re moving another step closer to being able to do that now.
Hopefully, they will be right.
via…